FDA Signals Major Shift on Peptides and It’s Sooner Than Anyone Expected

A quiet but explosive moment just happened in the peptide world.
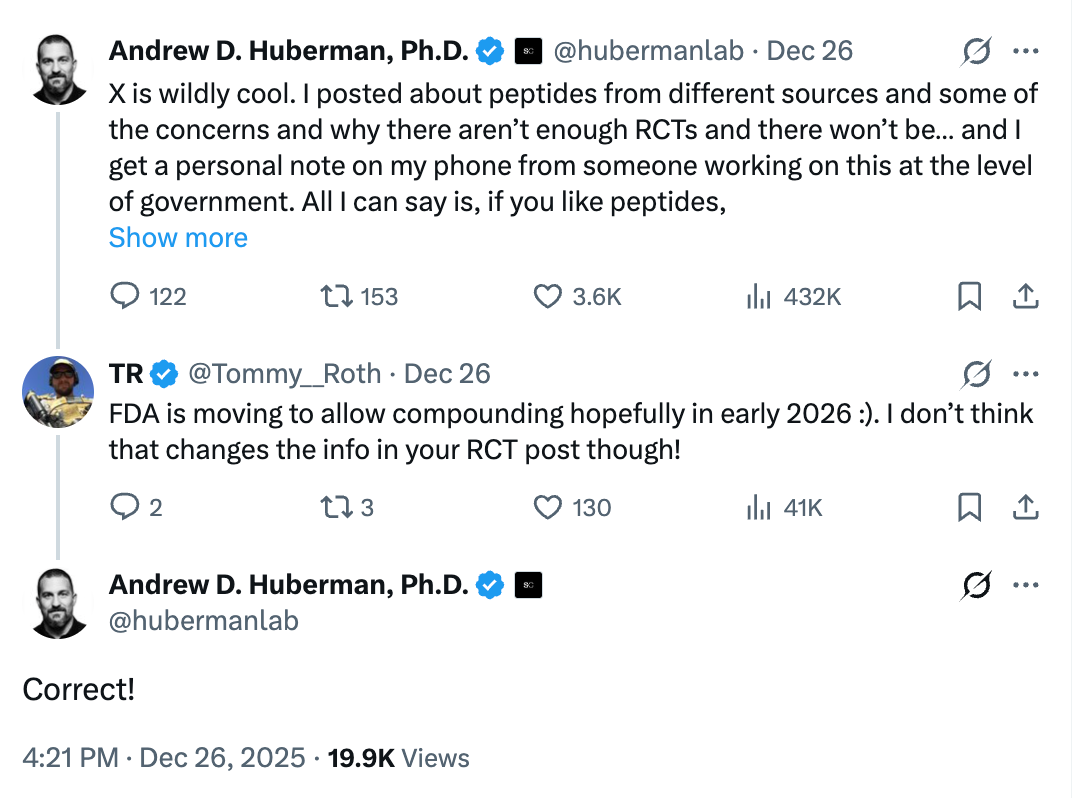
Andrew Huberman weighed in on the growing conversation around peptides, randomized controlled trials, and regulatory limitations. His takeaway was familiar to anyone paying close attention: the science is promising, but large-scale RCTs have been limited and likely will remain that way.
Then something unexpected happened.
A reply from X user @Tommy__Roth cut through the noise:
“FDA is moving to allow compounding hopefully in early 2026 :)”
Huberman’s response?
“Correct!”
If accurate, this signals a meaningful regulatory shift that could reshape access to peptides in the United States far sooner than most people anticipated. For years, peptides have lived in a gray zone, widely discussed, increasingly used, but constrained by regulatory uncertainty. This exchange suggests that change may already be in motion behind the scenes.
To be clear, this does not magically solve the RCT problem. It does not turn peptides into FDA-approved drugs overnight. But it does suggest that regulators may be preparing a more pragmatic framework for compounding, one that acknowledges real-world use while balancing safety and oversight.
For anyone following peptides closely, this is one of the most bullish signals we have seen to date.
Early 2026 is not far away.
And when people at the highest levels are publicly confirming these conversations, it usually means the groundwork is already being laid.
If you care about peptides, pay attention. This is how big shifts start.